| Democrite, thinker of the Vth century BC, taking back to his master Leucippe talks, suggest an atomic theory: | Matter is made from non-divisible particles ("atomos" in greek). |
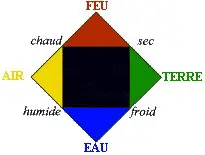
 | In the IVth century BC, Empedocle, contrarily to Democrite talks suggest this theory: | Universe is made from four elements :
|
 | At the same periode, Aristote strength the theory by adding four qualitys to the four elements: cold, dry, hot, wet. Those qualitys in couple form elements, who form the universe. This theory reach to explain some freaks of nature. | 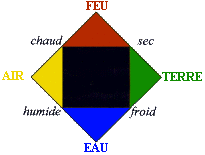 |
| 23 century later... | ||
 | In 1808, John Dalton, chemist and physician, rewrite and make deeper atomic theory of democrite: |
|
 | In 1869 Dimitri Ivanovitch Mendeleïev, chemistry professor in St-Petersburg classify elements according to their propertys. Writing a chemistry book, he discover that sorting elements by their atomic mass, a cyclic trend of their propertys appear several times. | |
 First Mendeleïev table | Mendeleïev make his table so that periodicity appear clearly, but to respect his periodicity law in which he trust, he must sometimes modify order and let some cells empty. He was sure that those missing elements will be finally discovered, and so predict the properys of those futur elements (according to the adjacents cells in the table): eka-aluminium, eka-bore and eka-silicium. Between 1875 and 1886, those three elements (gallium, scandium and germanium) where discovered. Each of them had the property predicted by Mendeleïev. | |
How to read the table
The horizontal rows in the periodic table form the periods. The electrons of elements in the same period are distributed over the same number of electron layers, a number given by the period number.
The vertical columns form the families. Elements belonging to the same family share certain characteristics.
This means that similar chemical properties recur periodically, hence the name periodic table.
Here are the names of the four main chemical families:
- The alkalis are at the far left of the table. All the elements in this chemical family have in common a very high reactivity to non-metals and water. When they react with water, they form an alkali, hence their name. They are soft, light metals with a silvery appearance. They do not occur pure in nature; they are always combined with other elements.
- Alkaline earth metals are on the right-hand side of the alkali family. They have alkaline properties in solution and are found in many rocks. They are metallic-gray solids. They are similar to alkalis, but harder and less reactive.
- Inert gases or noble gases are located in the last column of the periodic table. All these elements have almost zero chemical reactivity with other elements. Their saturated electronic layers make them highly chemically stable. Colorless in their natural state, they emit characteristic colors in vacuum tubes.
- Halogens are in the left-hand column of inert gases. They are so reactive that they are only found in a combined state in nature. This family takes its name from a Greek word meaning "salt generators". In fact, they form salts with alkalis, and produce strong acids with hydrogen.
Hydrogen is above the alkali family. In fact, it belongs to no chemical family at all. It is a unique element that can behave like an alkali and sometimes like a halogen.
Other chemical families are designated by the name of the first element at the top of the column on the periodic table. You can also use the Roman numeral and the letter (a or B) that appear at the top of the table's columns.