This site uses only a few technical cookies necessary for its operation. By continuing to browse, you accept their use.
To find out more...
To find out more...
The (small) miracle of béchamel sauce

Making a béchamel sauce is going to confront you with a little miracle that happens every time: You pour milk over a roux, it's very liquid, you stir over a low heat, and then all of a sudden, miracle, the sauce sets, it thickens, you've got your béchamel.
Let's see what happened.
Let's see what happened.
9,048 4/5 (4 reviews)
Keywords for this post:SauceMethodPrincipleExplanationStarchTemperatureRouxConsistencyBéchamelLast modified on: August 27th 2024
The (small) miracle of béchamel sauce
A basic sauce

Making it is quite simple: first a "roux" mixture of flour and butter, which is heated and colored (hence its name).

Once the roux has reached the right color, cold milk is added, the mixture is very liquid, and you continue to cook and stir over low heat until it thickens (you'll find the full recipe here).
But why does it thicken? As you'd expect, there's no magic involved, just a little physics and chemistry.
Flour and starch
The secret lies in the roux's flour, which contains grains of starch, a complex sugar made up of glucose molecules rolled up into tiny little balls.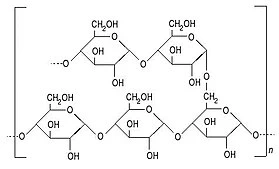
It's around 70°C that the magic happens: the grains of starch in the flour break up into smaller grains, starch molecules that begin to absorb the milk around them, up to 20 times their volume, thus increasing the sauce's viscosity.
This process, known as starch gelatinization, takes place between 70°C and 85°C, giving béchamel its characteristic thick, creamy consistency.
Note in passing that this process of starch gelatinization is also at work in, among other things, crème pâtissière or flan, where flour is often replaced by maïzena, a corn starch.
In short: Béchamel thickens because it contains flour that is heated, causing its starch grains to burst and absorb the milk around them.
So béchamel is not just a culinary classic, it's also a little chemistry lesson in action.
Lasts posts
XO Cognac Explained: Meaning, Aging, and Flavor Profile
XO Cognac always goes beyond the labels on the bottle: it is often associated with tradition and quality. You get to appreciate the artistry, character and ageing process when you understand what defines this smooth Cognac. The section below tackles everything about XO Cognac, from complex flavour...January 28th 20261,122 Sponsored article
Butter vs. grease
We often read in a recipe where a pastry is put into a mould that, just before pouring, the mould should be buttered or greased. But what's the difference between these 2 terms?December 1st 20252,6925
Getting out of the fridge early
Very often when you're cooking, you need to take food or preparations out of the fridge, to use them in the recipe in progress. There's nothing tricky about this: you just take them out of the fridge and use them, usually immediately, in the recipe. But is this really a good method?November 24th 20251,7215
Who's making the croissants?
When you look at a bakery from the outside, you naturally think that in the bakery, the bakers make the bread, and in the laboratory, the pastry chefs make the cakes. It's very often like that, with each of these professions having quite different ways of working, but sometimes there's also one...November 23th 20251,570
Oven height
When we put a dish or cake in the oven, we naturally tend to put it on the middle shelf, and that's what we usually do. But in some cases, this position and height can be a little tricky, so let's find out why.October 8th 20255,3635
Other pages you may also like
Soup vs. potage
It's true that we're finally coming out of winter as I write these lines, and that we'll all be making, no doubt, a little less soup and potages, but even if it's out of season, it really is a simple and delicious dish, which is one of the always easy answers to "What's for dinner this (Sunday)...April 9th 202212 K
Fruits which can ruin your jelly
There are many ways of making a fruit mousse, but one of the simplest is to prepare a fruit jelly (basically a fresh fruit coulis with gelatine) and then mix this jelly before it sets completely with whipped cream. The result is perfect for filling a charlotte, for example. But do beware;...March 6th 201379 K4.0
The window-pane test in bread-making
The home bread-makers often ask themselves “Have I kneaded my dough long enough?” . A good question, as dough that is insufficiently kneaded will not rise properly or will fall flat when the top is slashed, which is very frustrating. To know when the dough is ready, one can rely on the length...June 16th 202197 K 23.9
Candied fruits: don't get ripped off
Do you like candied fruit? You might like to nibble a handful or add it to a recipe, like a classic fruit cake or delicious Italian specialities like panettone or sicilian epiphany pie.June 21th 201769 K 24.2
Thermal inertia or "out of the fire"
When you're cooking, you need a lot of heat to cook, and most of the time it's on the fire, literally if you're on gas, more indirectly if you're not. An expression that comes up quite often is "Off the heat", but what does it really mean?December 12th 202011 K4.6
Post a comment or question
Follow this page
If you are interested in this page, you can "follow" it, by entering your email address here. You will then receive a notification immediately each time the page is modified or a new comment is added. Please note that you will need to confirm this following.
Note: We'll never share your e-mail address with anyone else.
Alternatively: you can subscribe to the mailing list of cooling-ez.com , you will receive a e-mail for each new recipe published on the site.